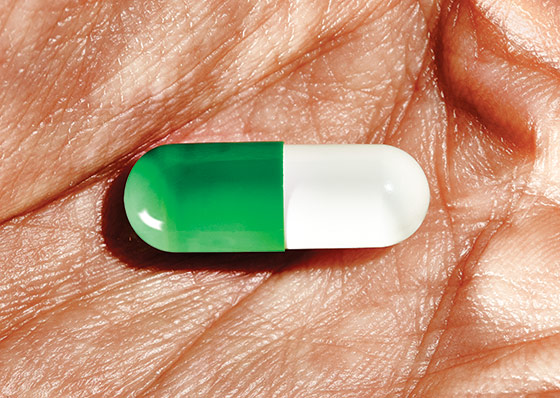
Even before a single doctor in the United States had written a prescription for Zohydro, the controversial long-acting painkiller approved by the Food and Drug Administration last October, potential users were already dreaming up possible street names. “How many times will this be said in the future,” someone posted on Opiophile, an online forum for people who like to share their drug experiences and expertise. “Got any of dem Zoh’s?” There were other possibilities: Zs, Zodros, and Zorros.
Another voiced chimed in: “I like Zorros … Yeah, has a ring to it.” This was on October 26, 2013, less than 24 hours after the FDA announced its decision.
And in April, even before Nima Majlesi, an emergency-room physician at Staten Island University Hospital, had seen a single report of an overdose or death related to Zohydro in the borough, he and his fellow doctor Amit Gupta were searching websites for the first anecdotal, and decidedly unofficial, accounts of its recreational use. Majlesi logged on to another well-known drug-use site to see if anyone had posted an opening-night review.
“How do you spell it again?” he asked Gupta, with whom he shares an unadorned office a few feet from the emergency room.
“Z-O-H-Y-D-R-O,” Gupta said, and Majlesi repeated the letters aloud as he typed them into a site called Erowid.
If the prescription-painkiller epidemic in America is a heartland phenomenon, as is often said, the heartland begins just beyond the toll plaza of the Verrazano-Narrows Bridge. As New York City Department of Health epidemiologists have helplessly documented over the past ten years, opioid abuse has skyrocketed on Staten Island, with three times the rate of overdose deaths of the rest of the city. In a borough where police have busted illegal prescription-drug-selling operations in everything from a neighborhood deli to an ice-cream truck, Majlesi and Gupta have seen and heard it all: the urgent calls of EMS teams phoning in reports of suspected overdoses as ambulance crews race to the hospital, lethargic or comatose patients (often young adults in their 20s and 30s) wheeled into the ER. Sometimes the overdose is accidental. Often there’s nothing to do because first responders didn’t arrive in time.
“No user experiences yet,” Majlesi announced, scrolling through the Erowid site. He tried checking Opiophile, but the site was blocked at the hospital. As far as he was concerned, it was only a matter of time. “I’m sure Staten Island will be among the first to see it,” he said glumly.
In the annals of new-drug rollouts, Zohydro seems to be in a class by itself. It has become a political nightmare for the drug’s manufacturer, Zogenix, Inc., and for the FDA—Massachusetts tried to ban it; the attorneys general of 28 states excoriated the FDA for approving the drug without “tamper-resistant” features, a decision Senator Charles Schumer of New York has called “baffling”; and Senator Joe Manchin of West Virginia has introduced legislation to roll back the approval. It has inspired apocalyptic warnings, mostly because Zohydro belongs to a class of drugs that the Centers for Disease Control and Prevention said in 2011 has created a nationwide, doctor-driven epidemic of addiction, death (roughly 16,000 a year), and unquantifiable familial devastation. And yet, so far, it has been nearly invisible—as of March 31, the company reported exactly 1,141 prescriptions filled nationwide.
“Watchful waiting” is a time-honored term in medicine, and it is the perfect phrase to describe the collective sense of anxiety, dread, and fatalism playing out as Zohydro slowly makes its way to pharmacies and ultimately into medicine cabinets. Zohydro is neither the first long-acting opioid painkiller nor, milligram per milligram, the most potent, so why all the fuss? Part of the concern is that because the drug is an extended-release formulation, it packs up to 50 milligrams of hydrocodone in a single capsule (Vicodin, the more familiar, instant-release version of hydrocodone, tops out at 10 milligrams per pill). And since it does not come in a tamper-resistant formulation, addicts can theoretically crush and snort or inject it to get an instant high from all 50 milligrams at once. OxyContin, the most infamous of prescription opioids and the main protagonist in the painkiller epidemic, did not come in a tamper-resistant formulation until 2010. By then, it had been implicated in thousands of overdose deaths since it hit the market in 1996.
It was precisely those fears that unnerved a panel of pain experts convened by the FDA to consider Zohydro 18 months ago. “Are we really, in the long run, helping people, or are we creating an epidemic?” asked one. As another briskly put it, “There are too many deaths already.”
Each year, the Drug Enforcement Administration, which tightly controls the amount of narcotic painkillers made in the U.S., sets the overall amount of opioid drugs that pharmaceutical manufacturers can churn out. In 2014, for example, the DEA total is 326,000 kilograms of opioids, including just under 100,000 kilos of hydrocodone. Put another way, that’s about 700,000 pounds of ersatz heroin usually doled out in 5 milligram or 10 milligram doses. Not all of that goes into domestic prescription medicine (some is used for research, some is shipped overseas), but since 2004, total amounts for oxycodone, hydrocodone, hydromorphone, and oxymorphone have basically tripled.

As a result, Americans consume 80 percent of the global opioid supply. “In most of Europe, it isn’t like physicians don’t prescribe opioids,” says Michael Von Korff of the Seattle-based Group Health Research Institute. “But they do it very cautiously, very selectively, and at low doses, and they don’t seem to be having the same problems we’re having here.”
The dramatic American embrace of painkillers in some ways begins with the distinction between acute and chronic pain. The former refers to the sudden, intense, and usually short-lived pain associated with a discrete event—surgery, a broken arm, getting your wisdom teeth pulled; it’s the kind of pain that typically requires a short course of painkilling medication, and virtually no one in the medical community disputes the utility of opioid drugs in this setting. Chronic pain, however, is an alternative universe of torment; if pain is, as C. S. Lewis once said, God’s megaphone, then chronic pain is a megaphone with a different kind of volume knob—incapacitating, demoralizing, life-altering. Among the more common causes are lower-back pain, migraine headaches, osteoarthritis, and fibromyalgia, a diffuse form of musculoskeletal pain, as well as cancer. As one patient told the FDA when it was considering Zohydro, his pain became so intense that “I told my doctor, ‘Either find me a tall building to jump off or something to help me with my pain.’ ”
But pain is very difficult to quantify for both subjective and psychological reasons. There’s no objective instrument to measure pain; patients typically rate their own (usually on a scale of zero to ten), but there is clearly a psychological component to its perception because, as FDA officials have pointed out, upwards of 40 percent of patients who take part in clinical trials report pain relief when given a sugar pill. For a long time, doctors shied away from using opioid painkillers for chronic pain, fearing the long-term side effects. In 1986, two doctors then at Memorial Sloan-Kettering Cancer Center, Russell Portenoy and Kathleen Foley, reported in the journal Pain that long-term opioid use in several dozen chronic-pain patients provided “safe, salutary, and more humane” treatment for non-cancer pain; the majority of patients said they achieved satisfactory pain relief, and the researchers reported very little risk of addiction (two patients out of 38, both with a prior history of drug abuse). It was the avatar of a message that would gain strength in the 1990s: Doctors were being excessively cautious about the use of narcotic painkillers, especially in cancer patients facing end-stage disease. A new, derogatory word trickled into the medical vocabulary: opiophobic.
In 1996, Purdue Pharma introduced OxyContin, an extended-release version of oxycodone for chronic-pain patients; it contained up to 80 milligrams of oxycodone (Zohydro contains up to 50 milligrams of hydrocodone, which many physicians believe is less potent and addictive than oxycodone). In marketing its product, Purdue deliberately downplayed the risk of addiction. Purdue Frederick Co. (part of the pharmaceutical company) and three company executives would later admit to federal charges of “falsely claiming that OxyContin was less addictive, less subject to abuse, and less likely to cause withdrawal symptoms than other pain medication.” (The company agreed to pay a staggering $634 million fine.)
That part about it being less addictive? Not quite true. “An opioid is an opioid is an opioid,” says Lewis Nelson, an NYU professor and attending physician in the emergency medical department at Bellevue hospital. “Heroin is illegal; oxycodone, hydrocodone, hydromorphone are all just legal versions of the illegal drug heroin from an end-user’s perspective.” And in part because of the ready availability of opioid painkillers, the National Institute on Drug Abuse estimates that 1.9 million Americans have become addicted to prescription painkillers.
In November 2011, the Centers for Disease Control and Prevention confirmed an “epidemic” with damning statistics showing that rates of opioid prescription, addiction, and fatal overdose rose virtually in lockstep since the late 1990s. “By 2010,” the CDC reported, “enough [opioid painkillers] were sold to medicate every American adult with a typical dose of 5-mg. of hydrocodone every four hours for one month.”
If there was a modest bright spot, it was that Purdue Pharma introduced a tamper-resistant formulation of OxyContin in August 2010; anyone trying to crush the pills to abuse the drug would end up with a gooey, uninjectible gel. Such measures seemed to discourage drug abusers from using the long-acting painkiller with its big payload of narcotic: A 2012 report in the New England Journal of Medicine documented a dramatic decline in OxyContin use among drug abusers after the change. But the report also detected an early hint of an ominous trend. Hooked on opioids, drug abusers began to move on to a cheaper and more plentiful alternative: heroin.
That same year, after extensive consultation with the FDA, a company in California submitted its application to market yet another high-dose, long-acting opioid painkiller. To the puzzlement of many, the FDA did not require Zogenix, Inc., to create a tamper-resistant form of the drug (the agency now says “the science is still evolving”). That’s when people like Lewis Nelson began to fear that Zohydro, as the new drug would be called, might be OxyContin all over again.
Sharon Walsh began to hear about the prescription-painkiller problem more than a decade ago, when she was studying IV-drug addicts as a researcher at Johns Hopkins University School of Medicine. She figured she should read up on how addictive the legal narcotics were compared with heroin and was stunned to discover that nobody really knew. “I realized that some of the fundamental questions had never been asked or answered,” Walsh recalls. She was particularly interested in hydrocodone. It is the most-prescribed drug in the country (roughly 130 million prescriptions a year), and physicians dispensed those prescriptions believing that it was less potent and less addictive than oxycodone. “But I really couldn’t find evidence for that,” she says. So she started to do rigorous, blinded studies of a drug that Americans had been using for decades, first in Baltimore and later in Kentucky, when she took a job in 2005 at the University of Kentucky. As she soon discovered, the eastern part of that state “happened to be the epicenter of the prescription-drug-abuse problem.”
Walsh has since become an expert on legal opioids and has served on FDA advisory panels. When the FDA began to consider tighter restrictions, the agency asked her to assess how addictive hydrocodone, the opioid in Zohydro, was compared with morphine and oxycodone, the active ingredient in OxyContin.
These “abuse potential” tests have a set of metrics all their own: After recruiting volunteers with “appropriate drug-use histories,” Walsh and her colleagues dosed them with hydrocodone, oxycodone, and morphine in both pill form and IV. While the subjects were high, the researchers measured “pupil diameter” (opioids typically cause “pinpoint” pupils) and rate of breathing (opioids suppress breathing, which is how most overdose victims die). They asked subjects to rate the drug’s “likability” and to estimate its street value. They even gathered data on something called “coasting,” which Walsh described as “a relaxed, calm feeling that is recognizable by opiate abusers.” By the time her lab gathered all this data, Walsh had reached two conclusions: In the view of drug addicts, hydrocodone was “virtually identical” to oxycodone, contrary to what many doctors thought. And if a new hydrocodone product got to market, she said, “it might produce problems just like oxycodone.”
Walsh noticed another paradox: the lack of evidence for the effectiveness of long-term opioid use, which other pain experts had also zeroed in on. Writing in the British Medical Journal, David N. Juurlink, head of clinical pharmacology and toxicology at the Sunnybrook Research Institute in Toronto, and two colleagues made the following point: “Many physicians are unaware that there is no evidence from randomised controlled trials to support the popular assertion that the benefits of long-term opioid therapy outweigh the risks.”
That wasn’t the way the manufacturer saw it. Zogenix argued that its product would fill a vital niche for chronic-pain patients. It served an “unmet need,” offering an alternative to existing painkillers, and added one more option for “opioid rotation,” the practice of switching to another painkiller when patients stop responding to their current medication. It would come in a long-acting formulation, so patients would only have to take two pills a day instead of four or six Vicodin. Perhaps most important, it would be the first long-acting hydrocodone that didn’t have acetaminophen, the active ingredient in Tylenol. As the company pointed out in its FDA application, many chronic-pain patients couldn’t get sufficient relief from Percocet or Vicodin because they came packaged with acetaminophen, which can cause liver toxicity and produce its own fatal overdoses.
Those issues set the stage for a compelling debate when the FDA’s Anesthetic and Analgesic Drug Products Advisory Committee met on December 7, 2012, to consider Zohydro.
There is a lovely bureaucratic Kabuki that plays out whenever the FDA convenes one of its advisory-committee meetings. During these daylong public sessions, the “sponsor” (the drug company) arrives with a cheerful army of personnel to give a well-rehearsed presentation that makes its clinical data seem irresistibly convincing. An FDA official usually gives a crisp, dry, and often astute analysis of the data. A panel of outside experts—the “advisory committee”—picks at the data with polite, occasionally pointed questions to the drug-company representatives. The key moment occurs at the end of the day, when the advisory-committee members discuss the safety and efficacy of the new drug and then vote on whether the FDA should approve it. As countless news stories have noted over the years, the FDA usually follows the recommendations of these advisory committees but is not legally bound to do so.
Throughout the morning session, it appeared like a typical FDA meeting, according to people in the audience like Walsh—a dispassionate scientific discussion, lots of data and graphs, the typical back and forth, but no hint of a brewing insurrection. Zogenix had conducted two clinical trials of Zohydro to test the drug’s effectiveness and safety, and there was some polite disagreement about the results. One trial started out with more than 500 patients with lower-back pain (one-third dropped out before its completion), but when the company scientists analyzed the numbers, they concluded that the drug provided a statistically significant improvement in pain relief—0.5 points better pain relief on a subjective, 11-point scale—compared with a sugar pill. An outside expert, Judith Kramer of the Duke University School of Medicine, burrowed into the company data, however, and pointed out that the placebo effect was enormous—54 percent of people taking Zohydro claimed to be satisfied by the relief of chronic pain at the end of one study, she noted, but so did 35 percent of people receiving a sugar pill, and in general the pain patients felt worse after three months of treatment than when they began—just not quite a bad as the patients on placebo. Zogenix medical director James Breitmeyer called the results “robust and clinically significant”; one of the outside advisers described the effectiveness as “modest at best.” The company also outlined an elaborate “safe use” program to educate physicians, monitor prescriptions, and minimize abuse of the drug; many committee members remained unconvinced the safeguards would work.
But the first significant departure from the usual FDA proceedings occurred during a midday session, when members of the advisory committee passed around a Kleenex box to dry their eyes as they listened to public testimony from ordinary citizens. The FDA typically reserves some time for patient-advocacy groups that want the agency to approve a drug; this time, many of the speakers described how painkillers had destroyed their lives.
Avi Israel, of Buffalo, chronicled how his 20-year-old son, Michael, developed a “medically sanctioned addiction” to hydrocodone, which was prescribed for his Crohn’s disease. “On the morning of June 4, 2011, after being refused help by his counselor, Michael locked himself in my bedroom, put a shotgun under his chin, and pulled the trigger,” Israel said. Cheryl Placek, also of Buffalo, described the “worst day” of her life, when her 28-year-old son, Daniel, a Navy veteran with chronic back pain who had become addicted to hydrocodone and begun to deteriorate psychologically, eventually hanged himself at the Veterans Affairs hospital in Buffalo in January 2012; if Zohydro was approved, she told the FDA, “you are basically inviting people to take their own lives, or it will end their lives.” Daniel Busch, a psychiatrist at Northwestern University’s Feinberg School of Medicine, described how addiction to prescription painkillers led to the death of his son, Joshua, a college student; “I’m disturbed the FDA is even hearing the new drug application for Zohydro,” he said. Pete Jackson, the head of a citizens’ group opposed to the overprescribing of opioids, described the death of his 18-year-old daughter, Emily, who overdosed on a single OxyContin pill. (There were many ordinary citizens lobbying for Zohydro, too, with equally heart-rending stories about life with chronic pain. But almost all of their stories began, “Zogenix has paid for my travel today.”)
The most dramatic moment of these hearings often happens when the panel debates, and votes on, the merits of a new drug. And the simple explanation for how the FDA wound up approving a high-dose, non-tamper-resistant version of a highly addictive narcotic came out in that final discussion and boiled down to a single philosophical question: What is more important, adhering to an equitable regulatory framework for drug approval (and maintaining what the FDA called “a level playing field” for the company) or considering the larger issue of public health?
The neutral tone of the early part of the meeting began to break down, and one of the triggers was when Sharon Walsh was asked to clarify her research. Zogenix representatives then attempted to portray her results on the abuse potential of hydrocodone as part of a “cloud of misunderstanding” and challenged her suggestion that Zohydro could be another OxyContin. Panel members took note of the way Walsh rebuffed these efforts (“Stalwart,” one doctor later called it), and she noticed that the advisory committee became more skeptical about the drug, its questions more pointed. “I could just feel and hear from the questions that were coming from the committee that there was some surge,” says Walsh. “Opinions were beginning to harden. And I think that the company felt that shift.”
The FDA staff may have sensed it, too. At one point during the afternoon session, Bob Rappaport, head of the FDA’s Anesthesia, Analgesia, and Addiction Products section, reminded the advisory committee that it had to consider the Zohydro application within the narrow regulatory framework that legally governed all FDA decisions: Was the drug safe and effective for the “intended population”—that is, for chronic-pain patients? Did the company satisfy the same requirements for effectiveness and safety that previous opioid manufacturers had satisfied when they had sought FDA approval? “To do other than what I’m asking you today,” Rappaport said, “while no doubt heartfelt and in the best interest of patients and the public health, may not provide us with a level of guidance that will be useful as we make our final decisions” about Zohydro.
Almost every time a panel member expressed concern about the societal danger Zohydro posed, Rappaport walked the discussion back to the narrow question of whether the drug was safe for intended patients. “This question, the last clause in there is ‘for the intended population,’ is it safe for the intended population. I just wanted to remind everybody of that.” A few moments later, Rappaport again redirected the committee’s focus to chronic-pain patients. If addicts diverted the drug and abused it, he said, “it’s really not a problem for the intended patient.”
“It seemed like Bob Rappaport was getting frustrated with the panel,” says Andrew Kolodny, chief medical officer of Phoenix House Foundation, the substance-abuse treatment center, who also testified at the meeting. “He actually started to scold the committee at one point.” By the time it came for the final vote, the exchanges between FDA staff and outside experts became, in Walsh’s view, “a little spicy.”
If there was a single voice politely insisting on arguing that the panel—and the FDA—had a greater societal responsibility, it was probably that of Judith Kramer, the doctor from Duke. Earlier in the day, she had questioned Zohydro’s effectiveness, predicting that the drug would cause harm because of its addictive potential; by late afternoon, she distilled the rising frustration of the committee when she explained why she didn’t think the drug was safe: “It’s striking me, as I’m listening to people give their reasons, that this drug is, in a way, held to a lower standard because of all the other drugs that we’ve accepted [with] this kind of profile … This drug will almost certainly cause dependence in the people that are intended to take it.” Summing up, she said, “I realize there has to be a level playing field in terms of business practice, but the primary thing has to be the public health.”
Ultimately, the advisory committee agreed with Kramer. “It started with more people surprisingly saying ‘No,’ and then there was quite a bit of momentum for ‘No,’ ” says Jeanmarie Perrone of the University of Pennsylvania, who was on the panel. When the votes were all counted, the FDA’s outside experts had not just rejected Zohydro by a lopsided 11-to-2 margin, as has been widely noted. In two votes leading up to the final judgment, the committee considered the drug barely effective as a treatment for chronic pain by a narrow seven-to-six vote; and even when forced to consider the drug’s safety in the context of its intended patients, as FDA officials had insisted all afternoon, a nine-to-five majority of the experts deemed the drug unsafe. Alan Kaye, a Louisiana State University School of Medicine anesthesiologist and pharmacologist, was proud of the vote.* “I was excited to vote ‘No,’ ” he recalls. “I was feeling I was there entrusted to make an educated decision that had a lot of implications for the people of the United States. And I know I made the right decision.”
Kaye left the meeting thinking Zohydro had been shot down, and he wasn’t alone. But Rappaport may have foreshadowed the agency’s thinking when he told the panel that day, “We may not be able to act on all of your recommendations because of our regulations and the law.”
On October 25, 2013, the FDA announced it had approved the drug. The agency gave its rationale later in a statement: “In the case of Zohydro ER [extended release], we determined that the benefits of the product outweigh its risks.” (The FDA has taken a public beating ever since; every time the FDA defends its decision, the makers of Zohydro post it on the company website like an endorsement.)
Before the sun came up the day after Zohydro’s approval, an opiophile had posted this reaction online: “When a 50 mg Zorro hits the block, it’s gonna fetch a big ol’ price, I betcha. And it’s anti-abuse-proof. Hell, yeah, I can see the Tweens lining up at my klinik already.”*This article has been corrected to show that Alan Kaye is an anesthesiologist and pharmacologist, not a toxicologist.
The emergency room of the main Staten Island University Hospital sits on Seaview Avenue, just up the road from the waters of lower New York Bay. It is impossible to think about the pending landfall of Zohydro on Staten Island without recalling the days prior to the arrival of Hurricane Sandy. Everyone knew the storm was coming, but they didn’t know when exactly it would arrive, where exactly it would hit, whether it would be one more overblown forecast or worse than anyone could possibly have imagined. As it turned out, Sandy sent seawater surging half a mile inland, right up to the entrance of the ER.
As if awaiting a different kind of storm, physicians on Staten Island are hunkered down, wondering what will happen with the arrival of Zohydro. As recently as mid-May, longtime ER physicians in New York like Lewis Nelson were having a hard time determining whether the drug had reached local distributors or pharmacies.
But on Opiophile’s “Zohydro” thread, the first reports trickled in on April 10. “Swim [someone who isn’t me] has 50 10mg Zohydro ER,” someone wrote. “They are in capsules that are nearly empty but contain little white, bead-like particles. Anyone with experience with this? Suggestions as to how to maximize their effect?” Within hours, someone had replied, “I believe junkie code says you must try a new pharm using each ROA [route of administration] and then give a full MLA style report on the phile. So I would snort one, eat one, iv one, plug one, and umm don’t smoke one. And don’t do them all at the same time!” Even allowing for zonker-braggadocio, it wasn’t an encouraging sign.
Many primary-care physicians, including on Staten Island, are now refusing to prescribe prescription painkillers; some even have signs in their offices saying so. First responders on Staten Island now carry naloxone (Narcan), a drug that can immediately reverse the effects of opioid overdose. And Zogenix has insisted, as it did during the approval process, that it is targeting only a “small subset” of about 90,000 chronic-pain patients.
It is entirely possible that the intense public scrutiny of Zohydro will hinder overprescription of the drug; Nima Majlesi suspects problems might not arise for another two or three years, by which time tamper-resistant forms of long-acting hydrocodone might be on the market. His emergency-room colleague on the south campus of the hospital, James Kenny, tends to agree. Down on the more affluent South Shore of Staten Island, where an equestrian course begins one block away from the hospital on Seguine Avenue, Kenny has already moved on to the next stage of heartbreak in the opioid epidemic.
“Yesterday, we had a 16-year-old come in, lethargic, and we reverted her with Narcan. She woke right up, tearfully admitting that she’s been snorting heroin for months,” he says in his basement office. “Her mom had no idea at all.” The surge in heroin use on Staten Island, he adds, “absolutely” started with prescription-painkiller addiction. Kenny argues that all the drug-company education programs and government restrictions on drugs like Zohydro are fine and necessary. “But,” he says, “that’s closing the barn door after the horse is gone.”