Jennifer Filippelli, a dark-haired, dark-eyed 30-year-old from the Bronx, felt all along that she would be a strong transplant match for her father, who was in dire need of a kidney: She knew it, you could say, in her gut. “My father and I are a lot alike,” she says. “We look alike. We both love animals. And we’re both stubborn.”
That last characteristic proved to be the biggest hurdle in the way of her father’s health when testing confirmed that Jennifer was, in fact, almost perfectly compatible as a donor, her blood type and proteins dutifully in sync. Her father wouldn’t consider taking her up on her offer. “He didn’t like the idea of my being cut open for him,” she says. Months passed and still her father procrastinated, even as he continued to retch every morning, ultimately losing some 30 pounds as the effects of kidney failure set in and toxins flooded his body. Finally, Jennifer says, “I threw a tantrum. I explained to him he could be forcing me to live with terrible guilt if he didn’t survive.”
Even that got her only to the next crucial step, which was to have a surgeon explain how the procedure would be performed. For most of transplant history, to donate a kidney meant to accept, in exchange, a permanent ten-inch scar across the abdomen and a six-week stay in the hospital, plus weeks of recuperation. But now, as the beneficiary of the newest minimally invasive technique, Jennifer would be out of the hospital in two days, back at work within three weeks. As for her scar, it would be no more than two and a half inches long – maybe even invisible by next year’s swimsuit season. At last, her father relented. “I don’t think we could have convinced him to go forward otherwise,” says Jennifer, sitting up in her bed at Columbia Presbyterian Medical Center. Two days after losing a major organ, she looks absurdly healthy, her color high; she’ll check out later that day. A crumpled package of Entenmann’s cookies beside her bed looks like it’s been ravaged.
“I would have gone forward with the surgery no matter what,” says Jennifer. “But he wouldn’t have.”
Jennifer Filippelli’s story embodies, in many ways, the best of what minimally invasive surgery offers: tiny incisions that open up huge possibilities, transforming our expectations about going under the knife. Surgeons have long been typed as the cowboys of the medical profession, the ones who slash and burn (or cauterize), crack open ribs, lay their hands on the body’s vital organs. But increasingly, the city’s most respected operating-room practitioners – among them, Jennifer’s surgeon, Dr. Dennis Fowler – function more like Silicon Valley technicians, operating by remote control, peering at projections on a television screen instead of a gaping incision in the body.
Not surprisingly, these new surgeons have been much more popular with patients than they have been with dismissive – and competitive – fellow surgeons. They’ve been embattled within their profession since the techniques were developed a decade ago. “There’s a ten-to-one ratio of guys who would love to see us get run over by a truck tomorrow, because they don’t know how to do this,” says Dr. Richard L. Whelan, chief of colorectal surgery at Columbia Presbyterian. But the remarkable results of minimally invasive surgeries have finally become an irresistible force in the medical marketplace, inciting a fierce bidding war among New York hospitals for high-tech talent, and redefining the future of surgery. Eventually, five, ten, fifteen years down the road, prophesies Dr. George Ferzli, an innovative multi-disciplinary surgeon at suny Downstate on Staten Island, “general surgery as we know it will not be around.”
Most of the pain in traditional postop recovery comes not from the vital organs that are the objects of surgery but from the slicing of layers of skin and muscle tissue to reach them. To avoid the trauma, the new surgeons operate by inserting a series of tubes into small puncture holes. At the tip of one tube is a scope, or camera, that projects an image onto a television screen; another tube, in the case of what is called laparoscopic surgery (which refers to minimally invasive surgery of the abdomen), inflates the body cavity with gas, so the organs float apart and the camera can distinguish between them. Usually, two other tubes serve as channels through which surgeons insert long, spindly instruments with gadgets at their tips that sever, clamp, and staple. It’s a difficult new skill set for surgeons to master, and the learning curve is steep. But the advantages are enormous: less suffering for the patient, a shorter hospital stay – many patients leave within a day or two – and a quicker return to normal activity.
Once considered gadfly specialists (“Nintendo surgeons,” their skeptical, older cohorts called them), these doctors are becoming must-have assets for hospital divisions of all kinds: gynecology, neurosurgery, vascular surgery, oncologic surgery – any department whose doctors manipulate what lies beneath the skin. Even if the new technology is pricey – sometimes outrageously so – hospitals with dwindling resources see it as a way to attract the kinds of patients who will make up for their other losses.
“If you look back historically, there are advances that moved medicine along, like anesthesia or open-heart surgery,” says Dr. Thomas Riles, chairman of surgery at NYU Medical Center. “Those were things that allowed us to treat a whole host of people we couldn’t treat before. Minimally invasive surgery is an advance of that kind of magnitude. The bursts of energy and ideas and technology are coming so fast – we’re still just trying to sort it all out.”
Like most successful revolutions, the minimally invasive phenomenon spread two ways: slowly, then quickly. “When I went to France to learn how to remove gallbladders laparoscopically in 1989,” says Dr. Barry Salky, New York’s first practitioner of the technique, “my chief of surgery said, ‘You can go ahead and learn, but you’ll never do it in this hospital.’ ” In fact, Salky persuaded his boss at Mount Sinai to let him proceed, and his early experience paved the way for his hospital’s considerable expansion in the field: Right now, some 28 percent of Mount Sinai’s surgeries are performed with the new techniques. What started out with gallbladders has expanded to almost every part of the body: colon, lungs, kidneys, the spine, and so on.
But for most of the past decade, New York’s entrenched, academic hospital environment lagged behind while the more freewheeling community hospitals – in Nashville, Atlanta, Kansas City – forged ahead. “All these New York hospitals were being run by very good open surgeons who missed laparoscopic surgery at the beginning,” says Salky. “And they fought it. These people were trained ‘big surgery, big incision.’ Plus, who wanted to learn a new skill? It was demeaning.”
In defense of the surgeons who hold back, some may be waiting to dive in until the techniques are perfected. “We’ve had a hundred years of open surgery,” says Kenneth A. Kern, clinical professor of surgery at Hartford Hospital and an expert in laparoscopic medical legal issues. “We know what its limitations are. We’re only just starting to figure out all the problems with laparoscopy.”
But already the tide is turning, as a host of local high-end hospitals have wooed minimally invasive surgeons to stay competitive. NYU and Montefiore Medical Center have established new divisions of minimally invasive surgery in the past two years. Dr. Dennis Fowler, Jennifer’s surgeon, came to New York from Pittsburgh fifteen months ago to head up a new minimally invasive division at New York-Presbyterian Hospital. Mount Sinai’s new director of cardiac surgery, scheduled to arrive this month, is Dr. Lishan Aklog, a minimally invasive cardiac surgeon from Brigham and Women’s Hospital in Boston. Memorial Sloan-Kettering Cancer Center, which has hired five minimally invasive surgeons in the past six months, is aggressively trying to hire still more in the next half-year. Recently, Fowler says, “it’s really turned around – there’s a craze for everybody to get someone.”
Among laparoscopic surgeons, NYU has a reputation for resisting minimally invasive techniques, but it signaled a commitment to changing that when it hired Dr. Michael Edye, a wry Australian who was Barry Salky’s first recruit at Mount Sinai. As Edye, preparing for a laparoscopic kidney removal on a Tuesday morning in November, waits impatiently for the anesthesiologist, one of his residents, a young Harvard graduate, offers up his theory of why minimally invasive surgery was relatively slow to catch on among doctors. “It was used early on by gynecologists,” he says, tying on his mask. “I think that has something to do with it” – prestigious surgeons, he speculates, were reluctant to take their cues from gynecology, long considered the least glamorous of the medical practices.
The atmosphere among the crew is light: By now, Edye has removed some 250 kidneys laparoscopically. In organ donation, the minimally invasive approach has proved to be an enormous boon: In one study done by the University of Maryland, the number of patients who had access to live donors rose from 12 percent to 25 percent over four years – a huge advantage, given the historical scarcity of organs for transplantation.
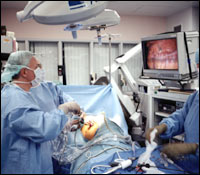
The patient, a young man visiting from Vietnam to donate his kidney to his brother, walks into the operating room smiling a bit self-consciously (he speaks no English) and lies down on the table. Five minutes later, he’s out, and the team prepares him for surgery, marking the site where the small incision for kidney removal will eventually be, then turning him on his side. After a few more minutes of preliminary stage setting, it’s lights out, so surgeons can better see the contrast on the television screens in front of them (this operating room has four). It seems appropriately womblike, as if to protect the internal organs from not only the knife but the startling brightness of the outside world. Four trocars – stubby plastic tubular pathways – are inserted, puncturing the skin, to provide access for the instruments.
Minimally invasive surgery is frequently described in terms of video games, but lined up alongside the patient, Edye and his two surgical-team members look more like foosball players: There’s that small, tight movement of the hands, their eyes somewhere else, the long levers releasing their surprising power. Using a series of 5- and 10-mm.-around, twelve-inch-long instruments inserted through the portholes, the surgeons snip at the connecting tissue attaching the colon, so that it falls away (the patient’s on his side, so gravity helps), allowing access to the kidney.
At no time, however, does something that looks like an organ in its entirety appear on the screen. Instead, the scope zooms in on individual connecting tissues, on tunneling, tubelike vessels, magnifying them up to twenty-fold, so that the geography is hard to assess for all but the carefully trained eye. “What I’m doing right now is a cross between flying and scuba diving,” says Edye, who has cut away at the fatty tissue surrounding the kidney and is moving on to the series of veins and arteries that will need to be severed to free up the organ. “It’s easy to lose one’s horizons.”
He won’t cut the most essential blood supplies to the kidney until the last minute, to preserve the health of the organ for as long as possible. When he does sever tissue containing blood vessels, he uses what’s known as a Harmonic Scalpel, an ultrasound device whose blades whir at a rate of 55,000 oscillations per second, simultaneously cutting and sealing vessels cleanly. At the site of the tool, the tissue bubbles at the point of severance and then blanches, closed off.
Edye, his eye still on the screen, corrects a resident controlling the scope, which also provides the light source in the body cavity: “Don’t weave around,” he tells him. “You’re moving the camera like you move your eye. We need smooth movements.” The directive sums up the challenges of the surgery, which requires a rethinking of all the basics: Move your hand to the right, and the instrument moves to the left; your surgical eye is not your own but the camera’s. Sensation, for novice surgeons at least, is limited. Edye, on the other hand, has a strong tactile feeling for his work. “It’s like using chopsticks – you can stroke the instrument over a structure and get a good appreciation for textures and fixation,” he says. “You feel it like a blind person feels the road with a stick.”
With the kidney nearly freestanding, Edye withdraws the instruments, the lights go back on, and the residents make a small incision, only about two inches long, in the young man’s side. Then the laparoscopic mode is reestablished. The lights dim again, and an instrument with a compressed plastic bag wrapped tightly around it is inserted through the incision. Through a series of careful pokes and pulls, it’s pulled off the instrument, then wrapped around the kidney. “It protects the organ when it’s pulled out,” explains one of the residents. “It also gives you something to grab on to.” Some two and a half hours after the surgery started, the kidney, gleaming and red, is pulled out intact and transferred to a waiting basin with ice. I half expect it to let out a wail.
Edye believes that laparoscopy is still in its early stages; every week brings new triumphs. “Last week, I took out a swollen nine-pound spleen – it was one and a half times the size of a football – through an incision about this big.” He spreads his thumb and forefinger apart. He used a technique called morcellation – the spleen is broken up into pieces inside a plastic bag in the body, then withdrawn bit by bit. What kind of surgery, I ask him, does he think could never be adapted to minimally invasive techniques? He thinks for a moment, then comes up with an answer: “Amputations.”
It’s nine o’clock on a Saturday morning, and Dennis Fowler has just sat down with oatmeal at Eli’s Vinegar Factory Café on the Upper East Side when his beeper rings. Fowler, who frequently bikes in the park with Edye – the circle of laparoscopic surgeons is small and close – is now director of New York-Presbyterian Hospital’s Minimal Access Surgery Center. As his meal, the only one he’ll eat until dinner, grows cold, he steps outside to return the call from an anxious father. Mustached and vested, a native of Kansas, Fowler looks and sounds like the kind of doctor who’d do house calls with a shiny black bag. Instead, he’s a high-tech gadgeteer, the first general surgeon to use the Harmonic Scalpel, a collaborator on the invention of some 25 or 30 surgical tools now on the market (though few were patented by him: “I wasn’t smart enough for that”). For Fowler, who most recently worked in Pittsburgh, one of his new job’s most glamorous perks is the chance to meet with Cornell and Columbia engineers. He started out working with engineers in 1990, when he needed a flat laparoscopic tool that would move a colon out of the way without puncturing it. Now he brainstorms with them about space-age designs, the possibility of a tiny freestanding robot that could operate inside the body.
Fowler’s mild midwestern manners probably served him well when hospital management was looking to hire its first director in charge of programs at both Cornell and Columbia, which recently merged – a tricky proposition politically. But Fowler’s no stranger to controversy: Shortly after he started using the new laparoscopic techniques at a hospital in Kansas, a colleague started harassing him. “He told me, ‘I will do everything I can to hurt you,’ ” recalls Fowler. “He tried to get me off the staff. He succeeded in dissuading doctors from referring patients to me. He threatened my children.” A certified medical professional threatened to harm Fowler’s kids? “He told me he’d catch them on the way home from school if I didn’t stop doing it,” says Fowler.
The story could be dismissed as an exaggeration, except that almost every other laparoscopic surgeon who started practicing in the early nineties has his own war story, some tale of sabotage, subtle or overt, from veteran surgeons. “They were threatened by it economically,” Fowler says simply, pointing out that gallbladder removal, for example, has always been the bread and butter of general surgeons. “It appears to me they could see the benefit of it but were afraid they couldn’t learn to do it. And if the benefits were really there and I could do it, I would take away all their cases.”
A decade later, financial issues are still roiling hospital departments: Take a colon-resection procedure Fowler pioneered in October 1990. Although cancer patients often prefer the laparoscopic procedure, because their recovery is swift and substantially less painful, the operating-room supply costs can be much higher – at NYU Medical Center, they’re nearly five times as expensive as open surgery. But the insurance reimbursement paid out to the hospital is no higher than it would be for the open surgery. Nor is the differential unique to colon resections. “Take an outpatient appendectomy – what we get from insurance doesn’t even cover the cost of the laparoscopic instruments alone,” explains Mona Sonnenshein, vice-president and senior administrator at NYU. And those instruments are largely disposable, used once and discarded.
Some of those higher costs, including operating-room time, will eventually plummet through experience and experimentation. (suny’s Ferzli has been known to forsake the Endocatch bag, a $139 plastic bag used to surround the kidney during organ removal, in favor of a two-cent Zip-loc baggie.) And already, argue the champions of the minimally invasive approach, hospitals should be recouping costs through shorter hospital stays, which net savings for hospitals since many insurance payers (including Medicare) reimburse them based on the diagnosis, not on the number of days a patient is on the premises.
But there is considerable disagreement on how to do the math in comparing the costs of new and older surgeries. Let’s say a new procedure’s complication rate is so low that a hospital knows it will have fewer return surgeries as a result – do you factor in that loss, perverse though that might be? Even the shorter hospital stays of laparoscopic patients are considered by some to be a suspect stat. “There’s a self-selection involved – the patients who are motivated to get back on their feet go for the surgery that’s reputed to have a quicker healing process,” says Dr. Stephen Gorfine, a surgeon at Mount Sinai who prefers performing open procedures. “And the minimally invasive surgeons are the biggest cheerleaders of all – You’re doing great, we’ll call a car, we’ll get you out of here tomorrow.”
Many hospitals seem to be heeding overwhelming patient demand, holding their breath and forging ahead with the procedures before the calculus is clear. “There’s spillover work that helps the bottom line,” points out Dr. Kenneth Abrams, medical director of perioperative services at Mount Sinai. If a patient comes to a given hospital for a particular surgery, that’s probably where he’ll go for all the other, moneymaking services: the diagnostic tests, the radiological services, and so on. If he’s pleased with the care he receives, he’ll come back. Says Abrams hopefully: “They’ll refer their friends.”
There’s a long-term concern at play as well: The more patients a hospital attracts, the stronger its negotiating position later on with the insurers. If it pays too much attention to costs and too little attention to patients’ wish lists, a hospital finds itself on the start of a perilous downward cycle, with fewer and fewer along for the ride.
Sherry Zubris, a secretary at Rutgers in her fifties, learned from her cardiologist in New Jersey that she needed to repair her mitral valve, which was swollen and impairing the functioning of her heart. The problem can be asymptomatic – and was, for the most part, in Sherry’s case – so it came as all the more of a shock when her doctor told her she would have to undergo open-chest surgery to fix a problem she had never even felt. Allowing herself to break down and cry as soon as she arrived home, she called her husband, Stephen, at work. Neither of them could imagine this young, healthy woman, who speed-walked with a friend every day at lunch, undergoing the agony of open-chest surgery.
Stephen Zubris spent the next three days researching possibilities on the Internet. Minimally invasive techniques kept coming up. The couple raised the procedure with their cardiologist, who only then mentioned a minimally invasive specialist to whom he sometimes referred his patients, a Dr. Stephen Colvin at NYU. “But if we hadn’t done the research, he’d have never even mentioned the possibility to us,” says Stephen.
At their doctor’s suggestion, the couple went ahead and met with the traditional surgeon he had first recommended. As soon as they asked that surgeon about minimally invasive procedures, says Stephen, still a little annoyed, “it was like a door slammed shut. He told us he doesn’t like to do things ‘half-assed.’ When we asked about cosmetics, he told us, ‘We try to keep the scar below the neckline.’ That was when we knew we’d be going somewhere else.” With Colvin’s procedure, a two-and-a-half-inch scar lies mostly unseen, hidden underneath the breast. It wasn’t just the scar they were eager to avoid but the six-week recuperation period, and the tremendous pain associated with healing.
Once they met with Colvin, it was “a no-brainer,” says Stephen, a bearded man who recounts the story in NYU’s sunny lobby on First Avenue. He’s waiting to hear the results of the surgery that Colvin ultimately performed. It helped that once they were given Colvin’s name, they found more than they could digest about him on the Internet, which is no accident. In a field where old-school surgeons are reluctant to refer patients to laparoscopic surgeons, a strong Web presence – financed, in some cases, by hospital public-relations dollars – is important in attracting potential clients (it also draws in relatively well-educated, wealthy patients, every doctor’s favorite kind).
In the operating room, where Sherry has just been anesthetized, everything is hushed and dim. Sherry herself is unidentifiable, her face covered in cloth, the rest of her covered in sterile orange plastic coating so that she looks more cyborg than human. Colvin has made a two-and-a-half-inch-long incision just beneath her breast. A rib retractor has pulled the incision and separated the ribs, so that the access site takes on a round shape, enough to fit three fingers or so through. Peeking surreally in and out of vision, as it pulses insistently amid all the plastic coating, is the left atrium of the heart, which Colvin will cut into to access the valve dividing that chamber from the ventricle below.
But before he can do that, he needs to stop the heart. Through incisions at the groin, he feeds one tube into the femoral artery and one into the femoral vein, following them all the way up to the heart. Those tubes are the lifelines to the heart-lung machine, which will take over the work of oxygenating Sherry’s blood. Inside the tube to the femoral artery, he threads a guide wire with a condensed balloon at the end of it; when it reaches the top of the aorta and is expanded, the balloon will block the flow of the blood.
As the room grows even quieter, the balloon is unfurled; that, plus a shot of solution containing potassium, stops the heart’s beating. The synchronized bleeping of a nearby electrocardiogram machine ceases. A startling slash of the heart, and Colvin has gained access to its interior, a view accessible to the room through the scope attached to his head. Its images are projected to the four TVs in the room, one of them the highest-quality HDTV available (four times the resolution of DVD, his technician says proudly). Up on the screen, the view is of exposed valve, fatty, yellow, and bulbous instead of flat and translucent, the characteristics of a healthy valve.
Dr. Colvin trims away the unhealthy parts of the valve and stitches together the remaining portions so they fit like a jigsaw puzzle, smoothly. All the knot-tying for the sutures happens at a five- or six-inch remove; each knot is then pushed down to the heart by an instrument called, appropriately, a knotpusher. As Colvin prepares to sew in a stabilizing band, it’s like watching someone do intricate embroidery at a five-inch remove from the canvas.
When the balloon is collapsed and retracted, and Sherry’s heart has been jump-started with a defibrillator, Colvin directs me to look at an ultrasound of the repaired organ. Before the surgery, the defective flap of the valve was curling back in on itself, and blood, instead of flowing down through it, was being thrown back up to where it had come from. The blood, represented by splashes of red on the screen, looked like the angry expulsions of a volcano. Now those flashes are gone; only a calm blue pulses in that area on the screen. Just under a week later, I call Sherry, who told me my timing was perfect: She was just finishing eating a full Thanksgiving dinner she and her family had delayed for a few days until her return.
For the past five years, alternatives to open-chest surgery have been expanding for some of the most common, and devastating, operations performed on the motor of the body. In the mid-nineties, Lenox Hill’s Dr. Valavanur Subramanian helped perfect a coronary-bypass method in which he manipulated one or two grafts through a small incision – without having to use the heart-lung machine, which studies have linked to risks of neurological damage. Since then, he’s performed hundreds of successful bypass surgeries this way. But it’s a practice limited, for the most part, to people who need only one or two bypasses; the need to manipulate three or four or five coronary arteries – in front, in back, on the side of the heart – usually precludes the possibility of an operation through a small incision.
Soon, heart surgeries could have doctors at an even farther remove, with robots doing the hands-on work. The million-dollar machines have enterprising names like Zeus and Da Vinci and are equipped with arms attached to surgical instruments no bigger than the joint of a finger. The surgeon sits at a console and manipulates hand instruments; the robot’s computer reads those movements and electronically transmits their parameters to the instruments at the end of the robot’s arms, which are calibrated to move analogously, only more minutely. The robot smooths out the tremors of a surgeon’s hands, which may eventually allow suture tying in small spots that would otherwise be inaccessible.
Colvin has used Zeus for several mitral-valve repairs but concedes that at this point, it’s more laborious than his current technique; at New York-Presbyterian, surgeons have repaired holes in the heart through entirely closed-chest surgery with the help of the robot; at Lenox Hill, Subramanian has experimented with the robot to perform single bypasses through an even smaller incision. Right now, the use of the robots is still experimental. But Subramanian hopes that within two years, using Da Vinci, he’ll be able to send single-coronary-bypass-operation patients home the next day. “That’s the goal of all this,” he says. Like every other pioneering minimally invasive surgeon I talked to, he quickly grows frustrated with the pace at which other surgeons are keeping up with the possibilities. “Cardiac surgeons are sleeping,” he says. “They don’t want to do anything different.”
Perhaps the only operations more traumatic than open-heart surgeries are those related to cancer. Columbia Presbyterian’s Richard L. Whelan is currently doing research on laparoscopic surgery that he thinks will transform cancer treatment. Whereas Fowler and Edye are understated, measured in their manner, Whelan is blunt: “Caveman surgery” is what he calls open procedures. “There’s a lot of animosity out there, a lot of bad feeling. But that doesn’t mean we shouldn’t move forward,” he says. “This is the way it’s going to be.”
It’s long been known that after major surgery, the body experiences a drop in immunological response for a week or so – the longer the incision, recent research has found, the more significant the drop. Whelan compared the results of patients who’d had procedures performed laparoscopically with those who’d had the surgery through a large incision and found that no comparable drop, or a much less significant one, occurred in the former. The results suggest there would be lower rates of infection in patients operated on with minimally invasive techniques, which is itself a noteworthy advance and supports many doctors’ clinical findings. But there are even more promising studies, on animals, that indicate that the chance of a tumor’s recurring after surgery may be greatly reduced with a small incision as opposed to a large one. “It means that if the surgery is performed closed, you’re better able to deal with tumor cells left over in a cancer patient,” says Whelan. “Those microscopic cells are the reason why a third to half of people who have surgery have recurrences. In open surgery, those cells are given a chance to run rampant right after the surgery. We think we can minimize that.”
Until now, the arguments in favor of laparoscopic surgery have been about immediate quality of life: cosmetics, the quicker recovery time, the reduced pain. But reducing pain may be more than just a luxury – it may somehow be bound up in the healing process. One recent study found that among rats with tumors, those given morphine had better survival rates. Surgeons aren’t sure whether it’s the length of the incision that’s most influential or the amount of exposure to microbes in the open air that the organs confront. But either way, “when you start talking about things that dramatically affect life span, then it’s game over,” says Whelan.
Even the most devoted of minimally invasive surgeons will admit that the procedures have their limiting factors, the most compelling of these being the skill of the surgeon. “Would I let someone who has taken out 25 colons laparoscopically take out my colon?” asks Mount Sinai’s Barry Salky. “No way.” The learning curve is both slower and steeper for laparoscopic surgeons, so patients are advised to ask carefully about the complication rates of a given doctor as well as the number of procedures he or she has performed.
Not surprisingly, as the number of laparoscopic procedures has risen over the past ten years, so has the number of malpractice suits filed: Between 1990 and 1994, there were 750 laparoscopic malpractice claims; between 1995 and 1999, that number ballooned to 1,426. The uptick raised enough eyebrows that in August 2000, the Physician Insurers Association of America published a study analyzing the most frequent missteps. As the report puts it, “Several of the claims reported indicated that the physician had trouble with visualization of the anatomical structures, which led to the physician dissecting or clipping the wrong duct or artery.” Given that it takes around five years for a malpractice claim to makes its way through the system, Lori Bartholomew, director of research for the PIAA, expects to see these numbers keep rising; and so far, the compensation rate has been higher for laparoscopic claims than with the average surgical-malpractice suit. “These are clear-cut injuries that sometimes require another surgery, or even lifelong treatment,” says Bartholomew. She adds that hospitals are starting to discourage laparoscopically assisted vaginal hysterectomies, not only because they take longer in the operating room but because “preliminary data suggests we’re seeing comparatively higher complication rates in that particular surgery.”
There’s no denying that closed surgery is more technically challenging than open surgery, although that perception (as well as malpractice rates) may change as medical schools step up the level of training they require for graduates. And it’s expected that younger surgeons more familiar with, yes, Nintendo may find the new techniques comparatively intuitive. “It’s not that endoscopic surgery is more difficult than open surgery but that it’s a different skill set,” says Mount Sinai’s Abrams. “People who might have been architectural engineers, automobile engineers – those folks would certainly enjoy doing this kind of surgery,” he says. “You’ll be drawing from a different pool.”
Already, it appears that the laparoscopic surgeons are getting a jump start professionally: Dr. Michel Gagner, the innovative chief of Mount Sinai’s prestigious minimally-invasive-surgery center, rose to that position at the tender age of 38. Dr. Ferzli – known as one of the most experienced general surgeons in minimally invasive techniques – says his laparoscopic fellows at suny Downstate are being offered starting salaries upwards of $50,000 higher than those available to their less-trained surgical counterparts. For two fellowship spots, he received 140 applications in 2001, up from 90 the year before. “And when I interview these candidates,” he says, “it’s like they’re desperate for the spots. The demand is so much higher than the availability.”
As more and more medical students come out of surgical residencies with new training, he predicts a generation of in-between doctors – the ones too young to retire but too well established to consider retraining – will be hard-pressed to continue with profitable practices.
“It used to be that a surgeon was like the symbol of man,” says Ferzli. “And we were like the feminine aspect of surgery – we were called wimps.” He savors the memory, then shrugs. “But we were right.”