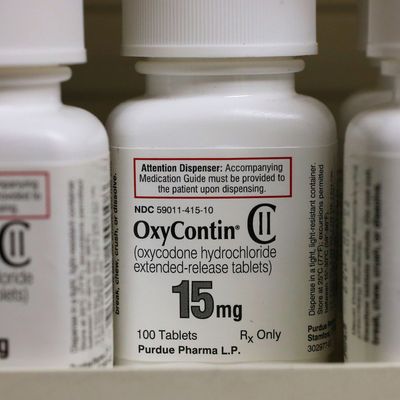
Like so many of our nation’s current crises, the opioid epidemic was born of a highly profitable lie: that extended-release opioid painkillers were far less addictive than the ordinary kind.
When Purdue Pharma set out to make a killing off of pain pills in the 1990s, two primary obstacles stood its way: Opioids were among the world’s oldest drugs, so low-cost generic versions of them were widely available — and most doctors believed that such painkillers carried a high risk for addiction and abuse.
With OxyContin, the drugmaker aimed to clear both hurdles at once. The drug’s novel, extended-release formulation could be patented — and then sold as a breakthrough that rendered OxyContin uniquely nonaddictive, and therefore, safe to prescribe for long-term use.
This was a clever play, but it suffered from one small flaw: There was little-to-no evidence to support support Purdue’s claim. Fortunately for the company, the Food and Drug Administration (FDA) gave Purdue the green light to say that OxyContin’s slow release was “believed to reduce” its appeal to drug abusers, who presumably favored a more immediate high.
In an internal 1995 report, Purdue hailed the FDA’s finding as “so valuable” that it could function as their new drug’s “principal selling tool.” Still, Purdue felt that the regulator’s approved language could use a bit of tweaking; instead of telling doctors and consumers that OxyContin’s formula was “believed” to make it relatively less appealing to drug abusers than Vicodin, Purdue advertised the drug as posing little-to-no risk of abuse and addiction, at all.
In truth, both the claim that the FDA approved — and the one that Purdue proceeded to make — were utterly false. The speculation that a slow-release formula would make OxyContin unappealing to drug abusers was irrational. Making a long-duration painkiller meant concentrating more narcotic into each individual pill. And since opioid addicts do not typically use pills as directed — but rather, crush them up for snorting or injecting — Purdue’s “innovative” opioid was actually more appealing as a street drug than any of its rivals.
The company’s top executives would eventually testify to Congress that they were unaware that OxyContin was being widely abused until the early 2000s. In 2007, Purdue and three of its executives pleaded guilty to federal charges of misbranding drugs — and were forced to pay a $635 million fine. By that point, Purdue had already made many billions of dollars off of OxyContin — and 29,600 Americans had already died from overdosing on the opioid.
But a Justice Department report newly obtained by the New York Times shows that Purdue was aware of its drug’s popularity with drug addicts and pill mills by the late 1990s, and that federal investigators wanted to charge three of the company’s top executives with felonies. Had those charges been brought, Purdue’s senior management could have found themselves in federal prison. But George W. Bush’s administration did not support the move, and prosecutors settled for pushing a misdemeanor “misbranding” case, instead.
Here is a sampling of what Purdue executives knew and when they knew it, according to Justice Department investigators:
Prosecutors found that the company’s sales representatives used the words “street value,” “crush,” or “snort” in 117 internal notes recording their visits to doctors or other medical professionals from 1997 through 1999.
“We have in fact picked up references to abuse of our opioid products on the internet,” Purdue Pharma’s general counsel, Howard R. Udell, wrote in early 1999 to another company official…[I]n 1998, as OxyContin’s marketing campaign was taking off, Purdue Pharma learned of a medical journal study that appeared to undercut its central message — that OxyContin, as a long-acting opioid, had less appeal to drug abusers.
In the study, which was published in The Journal of the Canadian Medical Association, researchers from the University of British Columbia in Vancouver interviewed local drug dealers and abusers to learn what legal drugs sold for on the black market. They found that MS Contin commanded the highest price of any prescription opioid with a 30-milligram tablet that cost $1 at a pharmacy bringing up to $40 on the street.
…Two years later, as OxyContin’s abuse publicly exploded in early 2000, a Purdue Pharma executive described in an email to Mr. Friedman, the chief executive, how he was reminded of what he had seen earlier managing MS Contin sales in the Midwest.
“I received this kind of news on MS Contin, all the time and from everywhere,” the company’s vice-president of marketing, Mark Alfonso, wrote in June 2000. “Some pharmacies would not even stock MS Contin for fear they would be robbed. In Wisconsin, Minnesota and Oklahoma, we had physicians indicted for prescribing too much MS Contin.”
Mr. Friedman’s response, prosecutors reported, was to forward that email to Mr. Udell with a question: “You want all this chat on email?”
The U.S. government believes that the narcotics trade is so morally odious — and societally damaging — it will give small-time crack dealers lifelong prison sentences for a first offense, and put tens of thousands of Americans behind bars merely for possessing illegal drugs. Currently, Attorney General Jeff Sessions is pushing to make prison sentences for drug offenses even more draconian, while the president routinely suggests that preventing the importation of narcotics to the U.S. justifies subjecting undocumented immigrants and asylum seekers to cruel and unusual punishments.
And yet, all the while, the government has allowed Purdue Pharma to collect more than $35 billion for engineering an opioid crisis that killed 42,200 Americans in 2016 alone — without attempting to put any of its executives in prison for their borderline-homicidal acts of fraud.
This failure to impose “law and order” on white-collar drug cartels has only emboldened them. While American doctors have soured on OxyContin in recent years — with prescriptions for OxyContin dropping by nearly 40 percent since 2010 — Purdue is looking to maintain profits by exporting our nation’s opioid epidemic to Europe and the developing world. All around the globe, Purdue’s international brand, Mundipharma, is working to overcome “opiophobia” — its term for foreign doctors’ mistaken belief that prescription opioids carry a high risk of abuse. As the Los Angeles Times reported in 2016:
For generations, physicians have been taught that opioid painkillers are highly addictive and should be used sparingly and primarily in patients near death. Mundipharma executives and consultants call this “opiophobia” and top company officials have said publicly that success in new markets depends on defeating this mind-set.
These tactics are nearly identical to those the company deployed in the United States in the mid-’90s. If the effects of that marketing prove identical, the results will be catastrophic; many of the developing countries that Mundipharma is targeting have far fewer resources than America does for providing drug treatment and rehabilitation.
Many progressives deride the “War on Drugs” as an utter failure. Some radicals disagree — arguing that it has actually been a smashing success, because the goal of the policy was never to deter drug abuse, so much as to keep anti-drug bureaucracies well-funded, and American prisons well-stocked with unruly workers that capital no longer has a use for.
When the government refuses to aggressively prosecute Purdue Pharma executives — while doling out life sentences to street-corner pushers — it lends credence to the the far left’s case.