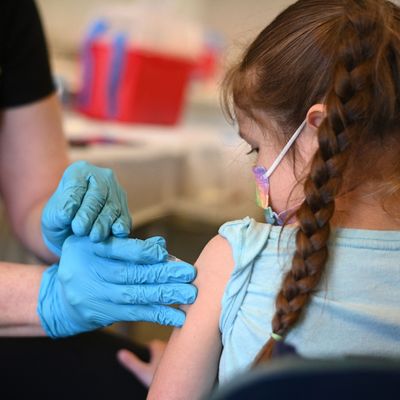
Earlier this week, Pfizer CEO Albert Bourla said that there was a “very high” likelihood that the Food and Drug Administration would authorize its two-dose vaccine for children under 5 at some point this month. But on Friday, the pharmaceutical company announced it would suspend its request for approval, holding off to wait for clinical trial data on a three-dose vaccine, anticipating that a third shot “may provide a higher level of protection in this age group.”
The delay was another blow to parents of young children still waiting for the vaccine: With the results expected in early April, it will now likely be several more anxious months before children younger than 4 and older than 6 months will be able to get their shots.
There was some prior concern from Pfizer that two doses would not provide an ideal level of protection for young children. In December, Pfizer and BioNTech started trials with three shots after data from the two-shot study found that it did not establish a strong enough immune response in some patients. The doses for the youngest age group are substantially smaller than those in older groupings: 3 micrograms for those under 5, compared to 10 micrograms for those 5 to 11 and 30 micrograms for everyone older than 12. The FDA was expected to publish an analysis of their data on Friday, with an advisory committee meeting scheduled on February 15, though that timeline has been suspended.
According to NBC News, there was some concern within the FDA regarding criticism from outside experts that Pfizer’s data was not enough for authorization and that these critiques were being ignored by regulators. Dr. Peter Marks, the head of the FDA’s branch responsible for vaccine safety, told reporters in a press call that the decision should give parents confidence that the agency is dedicated to assuring that the shots for tots are safe and effective. “Rather than having any issue of causing anyone to question the process, I hope this reassures people that the process has a standard, that the process is one that we follow, and we follow the science in making sure that anything that we authorize has the safety and efficacy that people have come to expect from our regulatory review of medical products,” he said. Most likely, however, it will cause frustration for the many parents who were ready to protect their kids in the second full year of the pandemic.