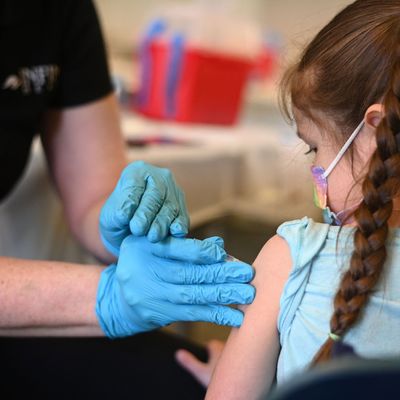
After months of delay, Pfizer has finally announced that it is seeking regulator approval for its COVID vaccine for children under the age of 5, which is great news for the millions of American parents concerned about their kids being exposed to the coronavirus.
The request for emergency-use authorization will now be considered by the Food and Drug Administration, whose vaccine advisory committee will review the submission on June 15. That day, the committee will also review Moderna’s EUA request for its COVID vaccine for children between the ages of 6 months and 5 years.
Unlike the adult-size version of the Pfizer vaccine, the protocol for kids over 6 months will now involve three shots, after a study found that it established an immune response in children similar to that in adults. Pfizer claims that a three-dose regimen in kids under 5 is 80.3 percent effective in preventing COVID infections with the study conducted after Omicron became the dominant variant in the U.S.
When Moderna submitted the EUA request for its vaccine for young children, it said clinical trials showed the two-shot vaccine was 51 percent effective at preventing illness in children ages 6 months to 2 years and 37 percent effective in kids 2-to-5 years old.
Pfizer’s shot for kids under 5 is just one-10th of the dose for adults, with the first two shots given 21 days apart and a third shot given at least two months after; in adults, that third (booster) shot is delayed until five months after the second dose.
Pfizer’s submission came after months of delay and frustration for parents hoping to give a level of protection for their young children. In December, Pfizer began trials for three doses after data for just two shots showed an insufficient immune response. In February, days after Pfizer CEO Albert Bourla said there was a “very high” chance the FDA would authorize a two-dose vaccine for the only remaining unprotected age group, the company suspended its EUA request to wait for the clinical data for three doses. During the first six months of Omicron, the only way for toddlers to develop an immune response to the virus was to get it.