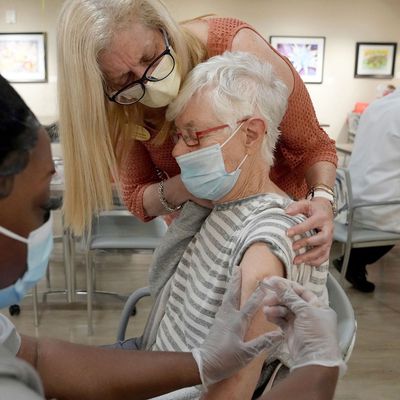
On Tuesday, the Food and Drug Administration authorized second COVID vaccine booster shots for any adults 50 and older who want to get one — though the agency is not officially recommending the extra booster, just making it available as an option. The move comes amid concern from some COVID experts about waning immunity among certain people who were boosted more than four months ago, as well as the possibility of a new wave of cases due to the BA.2 Omicron subvariant, which is more transmissible than its predecessor, BA.1, and has now become the dominant strain in the U.S. However, experts remain divided on the necessity of an extra booster at this time, based on the still-limited data about its benefits. Below is an overview of what we know thus far.
Who can get a second booster shot?
Anyone who is 50 or older is now eligible for a second booster shot, if they want one, and if it has been four months since they received their first booster shot. In addition, anyone with a weakened immune system is eligible for a second booster.
Who should get a second booster shot?
There is little consensus from the medical community on this question, mainly because there is pretty limited data regarding the benefit of the additional booster shot at this point. But those who do recommend getting the extra dose agree that it would be most useful for people who are at higher risk for severe disease from COVID-19.
Both the FDA and the Centers for Disease Control and Prevention said an additional booster should be made available to people who are immunocompromised, or 50 and older, if they’re four months past their last booster. This includes people in these groups who have received either three doses of some combination of the Pfizer and Moderna mRNA vaccines, or anyone who received the Johnson & Johnson vaccine as their initial dose and one dose of an mRNA vaccine as their booster. For immunocompromised people, who have already twice been offered a booster, this latest one may actually be their fifth shot.
Dr. Peter Marks, the director of the FDA’s Center for Biologics Evaluation and Research, said in a statement that “current evidence suggests some waning of protection over time against serious outcomes from Covid-19 in older and immunocompromised individuals,” and that based on emerging data, an extra booster “could help increase protection levels for these higher-risk individuals.” He also told reporters on March 29 that the FDA’s focus on people 50 and older was because one in three people between the ages of 50 and 65 has a health condition that puts them at higher risk of severe illness from COVID-19.
The bottom line? If you’re not sure, talk to your doctor about whether or not an extra booster shot would benefit you at this time.
What are other experts (and the data) saying?
As STAT News notes, the available research on second boosters is limited:
Health officials cited data from Israel showing that second boosters increased antibody levels, while other studies from Israel have shown that the shots increased protection against death during the country’s Omicron wave. Much of that data is considered preliminary, and it’s only been a few months since those doses started going into arms. Pfizer and BioNTech also said they submitted data to the FDA showing some waning of effectiveness three to six months out from the first booster shots.
It also remains unclear how long any extra benefit from the extra booster will last, including how long it will protect against infection.
The New York Times points out that for seniors, timing the extra shot well could be advantageous:
If another surge is just around the corner, for instance, seniors may benefit from getting an extra shot as soon as it’s authorized. But if the next wave doesn’t occur until the summer or even the fall, getting a booster now could backfire because the recipients’ immunity might start to wane by the time they need protection the most. Current vaccines are based on the original strain of the coronavirus, so getting a booster now may also do little to protect against future variants.
“It would be great if we knew exactly when the next wave was going to be so we could vaccinate people beforehand,” said Dr. Amy Sherman, an infectious disease physician at Brigham and Women’s Hospital in Boston. “But I think we’re not quite at the point where we know a clear seasonality, and we know the exact tempo and dynamics of Covid and newer variants.”
Scripps’ Dr. Eric Topol recommends a second booster shot “if you are more than 4–6 months from your third shot, you are age 50-plus, you tolerated the previous shots well, and you are concerned about the BA.2 wave where you live, or that it’s getting legs as you are trying to decide. Or if you are traveling or have plans that would put you at increased risk.”
Topol concurs that “the question is when is the right time, and whether an Omicron-specific vaccine will have any advantage over a second booster directed at the original strain,” though he notes that vaccines that have been updated to specifically target Omicron may not be available until May or June at the earliest.
Did the FDA or CDC consult their advisory panels of outside experts before ruling on extra boosters?
No.
Do people who have already had a booster and had Omicron need another shot?
It’s probably less pressing for that group, according to Dr. Topol, who recently noted that “you’ve got some hybrid immunity and you can save an extra shot, if or when there’s ultimately supportive evidence for a later time.” Dr. Bob Wachter, who chairs the University of California San Francisco’s department of medicine, likewise told STAT News that boosted people who caught an Omicron breakthrough infection could probably afford to wait a few months before getting another shot.
Will the U.S. government have enough funds or supply to get Americans a second booster?
The Biden administration has said it already has enough doses on hand to give second boosters to American seniors this spring. Beyond that, it’s not clear. The administration has repeatedly warned in recent weeks that Congress will need to approve more pandemic funding in order to make sure that every American can receive an extra dose for free.
Which vaccine boosters have been authorized?
The FDA authorized the Pfizer and Moderna mRNA COVID vaccines for use as second booster shots. Pfizer had requested authorization for second boosters for people 65 and older. Moderna had requested authorization for all adults.
Have the vaccines been updated or reformulated?
No, the additional booster shots will be the same formulations of the mRNA vaccines (designed to counter the original COVID strain) administered in previous rounds of vaccination.
More on Covid-19
- Murder Rates Are Dropping Despite GOP ‘Crime Wave’ Rhetoric
- Is a Sick Kid Better Than an Absent Kid?
- The GOP’s Claim That We Were Better Off in 2020 Is So Weird